Scout Bio’s mission: to harness the genetic revolution transforming human medicine to deliver the future of veterinary medicine
By combining world-leading talent in animal health and gene therapy technology, Scout Bio has built a development platform to address major areas of unmet medical need in companion animal (pet) medicine. Scout Bio is supported by leading life sciences investors and a partnership with the renowned Gene Therapy Program at the University of Pennsylvania.
Scout Bio: at the cutting edge of veterinary medicine
For the first time, a new generation of animal medicines may outpace human development — and revolutionize pet care in the process. The Scout Bio team’s mission is to lead the development of one-time gene therapies for pets, by capitalizing on:
- Strong demand for long-lasting off-the-shelf solutions for major conditions
- A critical mass of veterinary biotechnology and gene therapy expertise, brought together in Scout Bio’s founding team and a partnership with the Gene Therapy Program (GTP) at the University of Pennsylvania
The evolution of pet therapeutics
- Animals increasingly became companions with an emotional bond
- Pet owners drove demand for treatments
- High demand for better care through safe, longer-lasting and more targeted therapies
- Learnings from human biologics revolution applicable to animals through innovation
- Gene therapy research has delivered the first human therapies informed by over a decade of animal data
- In veterinary medicine, there is continued demand for therapeutic solutions with long-lasting action, providing safe and convenient care
Gene Therapy for Animals: Key Concepts
At Scout Bio, we’re focusing on delivering a new generation of therapies for pets to significantly improve the standard-of-care for multiple conditions. To achieve this, our technology approach is to express an appropriate level of a therapeutic protein in pet patients using adeno-associated viral (AAV) vector technology, delivered through simple intramuscular injection.
Targeting major health conditions in dogs and cats
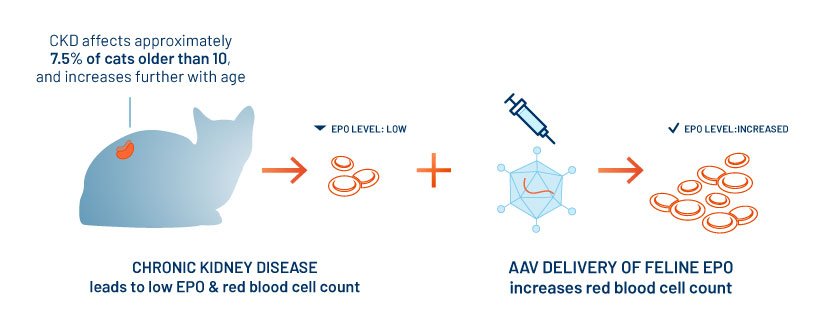
To date, while human gene therapy has focused on rare diseases, Scout Bio believes the opportunity in animal health begins with major areas of chronic illness where the long-term expression of a single functional protein could yield significant clinical benefits for an extended period from a single injection. This paradigm also promises significant advantages for convenience, owner compliance, and disease management. Scout Bio’s first therapy in development focuses on the anemia associated with chronic kidney disease (CKD) in cats. CKD is one of the most common health conditions in older cats. It progresses with age and can lead to death. CKD produces a range of other associated clinical signs, including anemia.
CHRONIC KIDNEY DISEASE (CKD) AND ASSOCIATED ANEMIA
CKD-ASSOCIATED ANEMIA
CLINICAL SIGNS: Anemia is a reduction in total red blood cell count. Progressive deterioration in quality-of-life with a variety of symptoms including weight loss, fatigue, weakness, fainting, and seizures.
INCIDENCE: CKD may affect younger cats, but is associated with aging. Approximately 7.5% of cats above age 10 have CKD, and incidence increases with age. Up to 65% of cats with CKD may have anemia.
CAUSE: Chronic kidney disease leads to kidney damage which reduces production of erythropoietin (EPO). EPO is required for normal development of red blood cells, which transport oxygen around the body.
DEFICIENT PROTEIN: EPO is a hormone produced in the kidney that stimulates red blood cell production in the bone marrow.
CURRENT TREATMENTS: There are no FDA-approved treatments for the anemia associated with CKD in cats. Iron supplementation is common and blood transfusions can be given. Treatment options include off-label recombinant human EPO injections, often multiple times weekly. These present logistical and financial challenges for owners and their pets. Furthermore, as the EPO is human – different to feline EPO – adverse immune reactions can occur.
STATUS OF SCOUT BIO PROGRAM: We are working towards delivering clinical proof-of-concept for the treatment of anemia associated with CKD via durable, feline EPO expression induced by AAV vector-mediated gene transfer. Our vector-delivered therapy candidate has demonstrated sustained and statistically significant increases in the red blood cell counts of cats, and we have commenced a large clinical pilot field study for this program in veterinary patients.

News
Ceva Santé Animale acquires Scout Bio, paving the way for groundbreaking innovations in pet therapeutics
Ceva Santé Animale acquires Scout Bio, paving the way for groundbreaking innovations in pet therapeutics
Libourne, January 25, 2024 – Ceva Santé Animale (Ceva), the #5 animal health company present in 110 countries worldwide, announces the strategic acquisition of Scout Bio, a pioneer in biotechnology focused on cutting-edge therapies for pets. The move represents a significant leap in innovation for Ceva, unlocking access to key advancements including a pipeline of monoclonal antibodies and gene therapy developments to address chronic diseases in pets.
While maintaining strong positions and continuing to develop biological and preventive medicine for all species, with this strategic acquisition Ceva aims to boost its global biotechnology activities, drawing on Scout Bio’s heritage but more importantly, fostering a future characterized by the acceleration of biotherapeutic innovation.
Scout Bio, renowned for its commitment to innovation, has been at the forefront of developing groundbreaking biotherapeutic products. This acquisition underscores Ceva’s recognition of Scout Bio’s nimble and responsive approach, as well as its excellence in clinical study execution in the biotherapeutics space.
Mark Heffernan, former CEO of Scout Bio, who has joined Ceva as Senior Vice President of Biotherapeutics and CEO of Ceva Biotechnology Campus (Philadelphia) expressed his enthusiasm by declaring:

Ceva has not only recognized our team’s delivery on innovation, but also our commitment to execution of quality clinical studies.
With the power of Ceva, we will be able to accelerate biotherapeutic innovation for the well-being of pets and the happiness of their owners.”
In a commitment to continuity, Ceva will maintain a strong relationship with the world-renowned Gene Therapy Program (GTP) at the University of Pennsylvania, from where Scout Bio originated as a spin-out from Dr. James M. Wilson’s lab. Dr. Wilson is director of the Gene Therapy Program, the Rose H. Weiss Professor and director at the Orphan Disease Center, a professor of Medicine and Pediatrics in the Perelman School of Medicine at the University of Pennsylvania, and a pioneer in the field of gene therapy. Together, Scout Bio and Penn’s Gene Therapy Program have jointly developed multiple programs in this emerging field, and Ceva aims to build further upon this success. The collaboration will progress existing programs and explore new product development opportunities where these types of disruptive technologies can be applied for the advancement of animal health.
“The recent acquisition of Scout Bio by Ceva marks a pivotal moment in the advancement of pet therapeutics. This integration of expertise, resources, and a shared commitment to innovation opens new horizons for Ceva. Indeed, with the growing trend of ‘pet humanization,’ owners aspire to extend the lives and well-being of their four-legged companions. This strategic alliance offers us the opportunity to develop products and solutions that cater to these expectations. Together, we are poised to lead in shaping the future of biotherapeutic solutions for the well-being of pets worldwide.” said Dr. Marc Prikazsky, Chairman and CEO of Ceva.
About Ceva Santé Animale
Ceva Santé Animale (Ceva) is the 5th global animal health company, led by experienced veterinarians, whose mission is to provide innovative health solutions for all animals to ensure the highest level of care and well-being. Our portfolio includes preventive medicine such as vaccines and animal welfare products, pharmaceutical solutions for farm and companion animals, as well as equipment and services to provide the best experience for our customers.
With 7,000 employees located in 47 countries, Ceva strives daily to bring to life its vision as a One Health company: “Together, beyond animal health”.
2022 turnover: €1.5 billion.
University of Pennsylvania Financial Disclosure
Dr. Wilson is a Penn faculty member, scientific collaborator, key advisor, and co-founder of Scout Bio. As such, he received an equity stake in the company, his laboratory at Penn received sponsored research funding from Scout Bio, and as an inventor of certain Penn intellectual property licensed to Scout Bio (now licensed post-acquisition to Ceva), Dr. Wilson may receive additional financial benefits in the future. The University of Pennsylvania also received sponsored research funding from Scout Bio, received equity in Scout Bio, and has licensed intellectual property to Scout Bio (now Ceva) that may result in future financial returns to Penn.
Scout Bio to Present at Upcoming Gene and Cell Therapy Summit
Scout Bio to Present at Upcoming Gene and Cell Therapy Summit
PHILADELPHIA, May 19, 2023 (GLOBE NEWSWIRE) | Scout Bio, a biotechnology company developing therapeutic proteins and one-time AAV gene therapies for major chronic pet health conditions, today announced that executive officers Mark Heffernan and Anne Traas, along with co-founder Matthew Wilson, will present at the 26th annual American Society of Gene and Cell Therapy (ASGCT) Summit in Los Angeles, California on May 20, 2023.
- Scout Bio Chief Executive Officer Mark Heffernan, Ph.D., will present an Overview of Animal Health Industry Similarities and Differences from Human Health. Saturday, May 20, at 8:11 a.m. PST.
- Scout Bio Chief Development Officer Anne Traas, DVM MS DACT, will discuss Disease Indications in Animal Health: One Medicine Approach to Gene Therapy. Saturday, May 20, at 9:55 a.m. PST.
- Scout Bio co-founder Matt Wilson will detail CMC Strategy – Decreasing Cost of Goods for Animal Health Market. Saturday, May 20, at 10:20 a.m. PST.
All three will take part in a panel discussion from 11am-12pm on Saturday.
About ASGCT
The American Society of Gene & Cell Therapy is the primary professional membership organization for gene and cell therapy. The Society’s members are scientists, physicians, patient advocates, and other professionals. Members work in a wide range of settings including universities, hospitals, government agencies, foundations, and biotechnology and pharmaceutical companies.
The mission of ASGCT is to advance knowledge, awareness, and education leading to the discovery and clinical application of genetic and cellular therapies to alleviate human disease. ASGCT’s strategic vision is to be a catalyst for bringing together scientists, physicians, patient advocates, and other stakeholders to transform the practice of medicine by incorporating the use of genetic and cellular therapies to control and cure human disease.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies and monoclonal antibodies for major chronic pet health conditions. Scout Bio’s therapies are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co.
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio Announces Appointment Of Chief Manufacturing Officer
Scout Bio Announces Appointment Of Chief Manufacturing Officer
- Experienced global animal health biologics executive to drive manufacturing strategies and advance pipeline of multiple animal health monoclonal antibodies (mAbs) and novel adeno-associated virus (AAV) therapeutics.
PHILADELPHIA, JULY 1, 2022 | Scout Bio, a biotechnology company revolutionizing pet medicine by delivering a pipeline of monoclonal antibodies and one-time AAV gene therapies for chronic animal health conditions, today announced the appointment of Ray O’Connor as its Chief Manufacturing Officer.
Mr. O’Connor’s unparalleled global technical leadership in novel biotherapeutics, process development, and technology transfer activities will advance Scout Bio’s robust portfolio to market. Mr. O’Connor brings more than 30 years of technical and leadership experience in human and animal health to this role, including senior leadership roles at Zoetis, Nexvet Biopharma, and Jazz Pharmaceuticals. In his role at Scout Bio, Mr. O’Connor will drive product development and manufacturing strategies.
“We are delighted to have an individual with Ray’s expertise to progress our monoclonals and single-injection AAV therapies into the key manufacturing phase and toward commercial launches,” said Chief Executive Officer Mark Heffernan, Ph.D. “This strategic appointment fills an important leadership role essential to delivering the next generation biopharmaceuticals in veterinary medicine.”
“Scout Bio has assembled one of the most advanced and broad pipelines in the animal health sector,” said Mr. O’Connor. “I am excited to join this highly motivated and proven team and to be part of the opportunity to deliver long acting and competitively priced novel biotherapeutics to owners and their pets.”
Mr. O’Connor’s CMC leadership experience spans three decades in the biopharmaceutical industry with senior global manufacturing roles in both animal and human therapeutics. Previous roles include Global Biologics Technical Leader at Zoetis, the world’s largest animal health company, a role he commenced after the 2017 acquisition of Nexvet Biopharma. During his leadership at Zoetis, Mr. O’Connor was a key contributor to the CMC development of Solensia, the first FDA approved animal health monoclonal antibody. He contributed heavily to the development of the monoclonal antibody manufacturing strategy for Zoetis, including the major expansion of the biopharmaceutical facility in Tullamore, Ireland. As Nexvet Biopharma’s VP of Technical Operations, Mr. O’Connor led the CMC strategy for animal health monoclonal antibodies.
Before joining Nexvet Biopharma, Mr. O’Connor held senior manufacturing roles at multiple human health companies including as the Head of Manufacturing at Jazz Pharmaceuticals, where he focused on designing and building internal manufacturing capabilities.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of monoclonal antibodies and one-time AAV gene therapies for chronic animal health conditions. Scout Bio’s therapies are designed to induce long-term expression of therapeutic proteins using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio To Present Positive Clinical Data On SB-001 For Feline CKD-Anemia at Leading Veterinary Medicine Forum
Scout Bio To Present Positive Clinical Data On SB-001 For Feline CKD-Anemia at Leading Veterinary Medicine Forum
- Scout Bio’s research was selected for presentation at the American College of Veterinary Internal Medicine (ACVIM) Forum
- Three lectures will present Scout Bio’s technology, new areas of research, and positive clinical results from first successful pilot clinical study with SB-001 for feline anemia
- First time a large multi-center study applying gene therapy to treat a chronic disease will be presented at a veterinary conference
Philadelphia, PA JUNE 14, 2022 – Scout Bio, a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies and monoclonal antibodies for major chronic pet health conditions, today announced that Scout Bio team members and clinical study investigators will provide presentations including information on Scout Bio’s veterinary gene therapies at the American College of Veterinary Internal Medicine Forum (ACVIM) 2022, which is taking place June 23-25 in Austin, Texas.
The presentations will discuss the benefits of single-injection adeno-associated viral vectors (AAVs), an emerging modality in veterinary medicine, and review Scout Bio’s AAV programs in development, including in the areas of feline anemia, feline and canine osteoarthritis pain, and feline and canine diabetes.
Lead author and clinical investigator in Scout Bio’s CKD-associated anemia clinical study, Dr. Shelly Vaden, will share positive data from the newly completed feline pilot study with SB-001, a product that has successfully shown proof of concept in this condition with significant unmet medical need.
With the potential of long-term efficacy from years to a lifetime after a single dose, AAV gene therapy may deliver an order of magnitude greater convenience and compliance for the management of chronic conditions in pets. “AAV is a platform that we believe will revolutionize the delivery of therapeutic proteins in chronic diseases in veterinary medicine and has the potential to change the way we will manage these diseases in our pets” says Scout Bio’s Chief Development Officer, Dr Anne Traas. “We are honored that the ACVIM has selected our research to present in a number of lectures at the conference, enabling us to share the potential for this platform to a wide audience of veterinary professionals, key opinion and industry leaders”.
The sessions featuring Scout Bio’s work at the ACVIM Forum include:
Adeno-associated Virus Vectors as a Delivery System for Treatment of Chronic Disease
Wednesday, June 22, 2022; 2:00 PM CT
Presenters: Cynthia R. Ward, VMD, PhD, DACVIM (University of Georgia) and Anne Traas, DVM, MS, DACT (Scout Bio)
Anemia of Chronic Kidney Disease, Pathogenesis and Potentials for Gene Therapy
Thursday, June 23, 2022; 3:00 PM CT
Presenter: Shelly L. Vaden, DVM, DACVIM (SAIM), PhD – (North Carolina State University College of Veterinary Medicine)
Novel Treatment for CKD Associated Anemia in Cats
Saturday, June 25, 2022; 10:20 AM CT
Presenters: Dr. Shelly L. Vaden
*Times are subject to change. The full agenda for the Forum can be viewed here.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies and monoclonal antibodies for major chronic pet health conditions. Scout Bio’s therapies are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio to Feature at Morgan Stanley Animal Health Biopharma Global KOL Summit
Scout Bio to Feature at Morgan Stanley Animal Health Biopharma Global KOL Summit
- Leading veterinary biopharma innovators, experts, and investors in gene therapy technologies, including Aaron Schacht and Mark Heffernan, to present opportunities for therapeutic innovation in rapidly growing market
PHILADELPHIA, June 09, 2022 (GLOBE NEWSWIRE) — Scout Bio, a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies and monoclonal antibodies for major chronic pet health conditions, today announced that it will feature at Morgan Stanley Animal Health Biopharma Global KOL Summit in New York City on June 10, 2022.
Chief Executive Officer Mark Heffernan, Ph.D., will present on the company’s background and strategy for delivering a new standard in care for chronic conditions in pets. “The veterinary therapeutics market has experienced strong growth in recent years. Innovation and dramatic increases in pet ownership seen during the pandemic are driving the forecasted growth acceleration,” said Dr. Heffernan. “Within this context, Scout Bio is uniquely poised to deliver that innovation through a diverse pipeline of novel biologic therapies, including AAV-based gene therapies designed to provide long-lasting efficacy for major conditions such as diabetes, pain, allergy and anemia.”
Scout Bio board member Aaron Schacht will provide industry-level context for the emergence of specialty biopharmaceuticals as a key growth area for veterinary therapeutics. Mr. Schacht is CEO of BiomEdit, a standalone venture recently formed through the carve-out of Elanco’s microbiome R&D platform and pipeline combined with the biology capabilities of Ginkgo Bioworks focusing on novel animal health product innovation. Elanco is the second largest animal health company in the world with over 10,000 employees and customers in over 90 countries, where Mr. Schacht formerly served as Executive Vice President with responsibilities over time including oversight of global research and development, regulatory affairs and business development.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies and monoclonal antibodies for major chronic pet health conditions. Scout Bio’s therapies are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio Appoints Life Sciences Executive Aaron Schacht to Board of Directors
Scout Bio Appoints Life Sciences Executive Aaron Schacht to Board of Directors
- Brings experience in managing global innovation portfolios and business development to Scout Bio
- Executive leadership experience across both animal and human health
PHILADELPHIA, February 18, 2022 – Scout Bio, a biotechnology company developing therapeutic proteins and one-time AAV gene therapies for major chronic pet health conditions announced it had appointed Aaron Schacht to its Board of Directors. Mr. Schacht is a deeply experienced life sciences executive with management expertise across a wide range of functions and will work with Scout Bio to execute its strategic development as a veterinary biopharma pioneer.
“Scout Bio’s approach to the innovation and delivery of entirely new biologic therapies for animals is truly unique. Their science is highly validated and has remarkable potential to deliver breakthrough treatments for chronic conditions in pets,” said Mr. Schacht. “I look forward to working with the entire Scout Bio team to support the advancement of the company’s business and rich pipeline.”
“I am pleased to welcome Aaron to Scout Bio during an exciting period where his expertise will add significant value to our corporate strategy,” said Mark Heffernan, Ph.D., chief executive officer of Scout Bio. “We are nearing several inflection points in 2022, including a number of significant clinical milestones for multiple programs. Aaron’s global product development experience will be invaluable in shaping our portfolio and supporting our mission to transform the treatment of major conditions with biologic therapies that will integrate seamlessly into veterinary care.”
Mr. Schacht was until recently an Executive Vice President at Elanco (NYSE: ELAN), with a number of responsibilities over time including oversight of global research and development and innovation as well as regulatory affairs and business development. Elanco is the second largest animal health company in the world with over 10,000 employees and customers in over 90 countries. Mr. Schacht currently serves as President and CEO of MBRD, a new company created to transform Elanco’s microbiome R&D platform and pipeline into a standalone venture.
During his time at Elanco, Mr. Schacht transformed Elanco’s innovation model, building and integrating internal and external innovation strategies that position Elanco as a leading independent animal health company. Aaron led the strategic acquisitions of Bayer Animal Health, Aratana Therapeutics, and Kindred Biosciences. His R&D teams delivered more than a dozen new products to the market during his tenure, while integrating the R&D capabilities and pipelines of Novartis Animal Health, Boehringer Ingelheim pet vaccines, Bayer Animal Health, Aratana, Prevtec and Kindred. Aaron was a member of the founding executive team that completed the carve out from parent Eli Lilly and Company, taking Elanco public in 2018. Schacht began his career at Lilly in 1990 as a medicinal chemist. He holds a Bachelors of Science degree from the University of Illinois.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies for major chronic pet health conditions. Scout Bio’s therapeutics are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio to Present at Upcoming Animal Health Conferences
Scout Bio to Present at Upcoming Animal Health Conferences
PHILADELPHIA, February 15, 2022 – Scout Bio, a biotechnology company developing therapeutic proteins and one-time AAV gene therapies for major chronic pet health conditions, today announced that it will present at two conferences in February 2022. They include:
- Animal Health, Nutrition and Technology Innovation Europe (In-person). Scout Bio is a sponsor of the event, and management will host a technical insights session reviewing the company’s platform and pipeline on February 22 at 3:15 p.m. GMT.
- Bank of America Securities Animal Health Summit (Virtual). Scout Bio Chief Executive Officer Mark Heffernan, Ph.D., will provide a corporate overview on Thursday, February 25, 2022 at 10:50-11:30 a.m. EST.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies for major chronic pet health conditions. Scout Bio’s therapeutics are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio Expands Gene Therapy Collaboration with the University of Pennsylvania Via Next-Generation Vector Technologies
Scout Bio Expands Gene Therapy Collaboration with the University of Pennsylvania Via Next-Generation Vector Technologies
- Partnership agreement includes exclusive rights in animal health to novel viral vector capsid and expands additional option rights to novel capsids
- Next-generation novel adeno-associated viral (AAV) vector capsid promises enhanced manufacturability
- Expanded partnership with Penn’s Gene Therapy Program continues to strengthen Scout Bio’s long-term leadership position in companion animal gene therapy
Philadelphia, PA December 7, 2021 – Scout Bio, a biotechnology company focused on one-time AAV gene therapies for major chronic pet health conditions, today announced the expansion of their foundational collaboration agreement with the Gene Therapy Program (GTP) at the University of Pennsylvania (Penn) to grant Scout Bio exclusive rights in the field of animal health to an emerging viral vector capsid technology for use in animal gene therapy, as well as extended option terms to other next-generation AAV vector technologies.
Scout Bio has undertaken a thorough evaluation of this new capsid, which is now the basis of several of Scout Bio’s programs undergoing in-species veterinary clinical trials. This capsid has been optimized for enhanced manufacturability to significantly increase yield per production batch. The next generation capsid may also deliver enhanced targeting of muscle cells, further supporting Scout Bio’s focus on gene therapy treatments that emphasize convenience for owners and ease of administration and integration into veterinary practice.
The collaboration expansion, which includes additional rights to novel capsids, leverages world leading AAV capsid discovery, development, and engineering programs under the direction of James M. Wilson, Director of the Gene Therapy Program at Penn and a co-founder of Scout Bio.
“This expanded collaboration supports Scout Bio’s continued scientific leadership in pet gene therapy and supports the manufacturing and commercial planning for our portfolio – which is already delivering meaningful clinical outcomes. We are proud to leverage the world’s leading AAV technologies to complement our in-house expertise in preclinical R&D, manufacturing, and clinical execution,” said Mark Heffernan, Ph.D., Chief Executive Officer at Scout Bio.
“Scout Bio’s complementary expertise means our partnership is ideally suited to deliver an entirely new paradigm to veterinary medicine and see transformational benefits in complex chronic diseases in companion animals,” Wilson said. “Scout Bio’s progress in veterinary patients is now showing significant potential of the AAV gene therapy platform for more expanded applications across multiple species.”
With three AAV product candidates in clinical development and the ability to move from concept to the clinic rapidly, Dr Heffernan said, “Scout Bio has built a discovery and development engine designed to meet the major needs in pet chronic disease with affordable, convenient, and durable one-time gene therapies.”
About AAV Vector-Based Gene Therapies For Pets
Scout Bio is the leading developer of gene therapies for pets. Each gene therapy candidate is a single injection designed to induce long-term expression of a therapeutic protein to treat a particular serious chronic veterinary disease or condition. Scout Bio uses a highly validated technology for the delivery of therapeutic genes called adeno-associated viral (AAV) vectors.
A ‘vector’ is a transporter for genetic material. AAV vectors are the most commonly used and studied delivery technology in gene therapy today. The exclusive rights covered in the collaboration agreement with Penn cover animal health applications of a new type of AAV vector capsid, which is the protein shell that mediates delivery of a vector’s payload to target cells. Capsid engineering can bring a range of additional benefits. For example, Scout Bio’s novel AAV vector capsids have delivered dramatic improvements in the manufacturability of the vector.
Scout Bio focuses on intramuscular delivery of gene therapies for pets to maximize convenience and integration into veterinary practice. The company’s data suggests that intramuscular delivery may deliver superior durability of therapeutic effect and mitigate any dampening effect of natural antibodies against AAV vectors. Scout Bio’s candidates are therefore optimized to specifically deliver therapeutic genes to muscle cells.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies for major chronic pet health conditions. Scout Bio’s therapeutics are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
University of Pennsylvania Financial Disclosure
Dr. Wilson is a Penn faculty member, scientific collaborator, key advisor, and co-founder of Scout Bio. As such, he holds an equity stake in the Company, his laboratory at Penn receives sponsored research funding from Scout Bio, and as an inventor of the licensed technology he may receive additional future financial benefits under licenses granted by Penn to Scout Bio. The University of Pennsylvania also holds equity and licensing interests in Scout Bio.
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio Named Best New Start-Up for 2021
Scout Bio Named Best New Start-Up for 2021
IHS Markit recognizes Scout Bio for pioneering therapies in companion animal medicine

Joseph Harvey, Head of Animal Health at IHS Markit, said: “Scout Bio’s progress has really stood out in 2021. It has a team of proven innovators that are again showing momentum in the companion animal therapeutics segment. This year, the start-up has shown significant development with both clinical trials and fundraising. The judging panel believe Scout Bio will be a key innovator of the future.”
Over the last twelve months, Scout Bio has accomplished a number of animal health industry firsts including the:
- First gene therapy in clinical development to successfully treat feline patients with an acquired non-monogenic disease (chronic kidney disease associated anemia)
- First to clinically advance a novel GLP-1 analog expressed via adeno-associated virus (AAV) gene therapy for feline diabetes in companion animal patients
- First vectorized monoclonal antibody to be studied in feline chronic pain when delivered by AAV gene therapy
“This award is welcomed recognition acknowledging our team’s innovative work in achieving a number of industry firsts amidst a year of COVID-related challenges,” said Mark Heffernan, Ph.D., Chief Executive Officer at Scout Bio. “I couldn’t be more proud of our team and key collaborators for their commitment to advancing gene therapy innovation in pet patients in a number of chronic diseases over this past year. In addition to these groundbreaking pipeline milestones, we are strongly positioned to continue to advance Scout Bio’s portfolio of AAV delivered therapeutic proteins for companion animal diseases thanks to the recent closing of additional financing.”
A spin-out company from the University of Pennsylvania (Penn) and the laboratory of Dr. Jim Wilson at Penn’s Gene Therapy Program (GTP), Scout Bio is uniquely able to leverage the expertise of GTP to accelerate discovery and development efforts in this exciting new field of companion animal medicine. “Scout Bio is on target to significantly disrupt the field by showing that AAV gene therapy can deliver therapeutic proteins as a single-injection alternative to weekly or monthly injectable proteins,” Wilson said. “Scout Bio’s progress in veterinary patients is now showing significant potential of the AAV gene therapy platform for more expanded applications in a multitude of species.”
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies for major chronic pet health conditions. Scout Bio’s therapeutics are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co
University of Pennsylvania Financial Disclosure
Dr. Wilson is a Penn faculty member, scientific collaborator, key advisor, and co-founder of Scout Bio. As such, he holds an equity stake in the Company, his laboratory at Penn receives sponsored research funding from Scout Bio, and as an inventor of the licensed technology he may receive additional future financial benefits under licenses granted by Penn to Scout Bio. The University of Pennsylvania also holds equity and licensing interests in Scout Bio.
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
Fran.gaconnier@scoutbio.co
Scout Bio Initiates Multi-Center Clinical Study of Gene Therapy for Chronic Arthritis Pain In Cats
Scout Bio Initiates Multi-Center Clinical Study of Gene Therapy for Chronic Arthritis Pain In Cats
– First vectorized antibody gene therapy to be studied in pets
– Strong initial in-species data support therapeutic profile of one-time gene therapy designed for long-term pain management
– Novel monthly pain management antibody therapy also assessed as part of study design
– Topline data expected in Q3 2022
PHILADELPHIA, October 28, 2021 – Scout Bio, a biotechnology company focused on revolutionizing pet medicine by delivering a pipeline of one-time therapeutics for major chronic pet health conditions, today announced the initiation of a clinical study to evaluate the safety and efficacy of SB-007, the company’s one-time AAV gene therapy for chronic arthritis pain in cats. After a single injection, SB-007 is designed to trigger consistent long-term production of an antibody that inhibits a pain-promoting protein called nerve growth factor (NGF) in cats. The study will also assess SB-011, which is the anti-NGF antibody encoded by SB-007, in parallel development as a monthly injection.
“Pet owners and veterinarians are looking for ways to relieve chronic pain and restore quality of life in the most convenient, long-lasting way, which is the promise of one-time gene therapies like SB-007,” said Mark Heffernan, Ph.D., Chief Executive Officer of Scout Bio. “Emerging anti-NGF antibody therapies have validated the potential of this approach, and as the leaders in pet gene therapy Scout Bio has a clear opportunity to significantly improve treatment options in this major therapeutic area, as we are also doing in anemia, diabetes and atopic dermatitis.”
The study is a multi-center placebo-controlled pilot clinical study that will target enrollment of 132 client-owned (pet) cats in veterinary clinics throughout the United States. The study will include an evaluation of the SB-011 antibody given monthly; or two combination groups where one dose of SB-011 is followed two weeks later by one of two dosages of the SB-007 gene therapy. The transition enables the direct assessment of anti-NGF activity and clinical outcomes delivered by a monthly antibody versus a one-time gene therapy. Endpoints will include validated owner-assessment tools for pain and mobility in cats. Topline data from the study is expected in the third quarter of 2022.
Both SB-007 and SB-011 target feline NGF which has been shown to play an important role in the production of pain signals and inflammation in diseases like degenerative joint disease (DJD). Inhibition of NGF is intended to dramatically reduce or eliminate pain, improving a cat’s quality of life and mobility.
“Our anti-NGF candidates represent another major innovation to emerge from our proprietary research and AAV development platform. This study expands our work in vectorized antibodies, a gene therapy approach that allows patients to produce their own therapeutic antibodies after a single, intramuscular dose of gene therapy,” said Matt Wilson, Scout Bio’s VP of Product Discovery and External Innovation. “Anti-NGF antibodies are only the first of many antibodies that may be vectorized through a design process where we have specialist expertise based on our foundational partnership with the University of Pennsylvania’s Gene Therapy Program. In addition to feline anti-NGF vectorized antibodies, we are also advancing a canine anti-NGF and an anti-IL-31 antibody program for the major area of atopic dermatitis.”
Data highlights from completed studies of SB-011 and SB-007 to-date include:
- SB-011: This monoclonal antibody (mAb) candidate has demonstrated potent neutralizing activity and repeat dosing in healthy cats has been shown to achieve a serum concentration with potential for therapeutic effect. SB-011 has also demonstrated favorable safety following multiple repeated subcutaneous injections. No immunogenicity has been observed.
- SB-007: Our gene therapy program to express our SB-011 monoclonal antibody has demonstrated the ability to produce sustained plasma levels of the monoclonal antibody within a target therapeutic concentration for over a year after a single injection. The animals in this pre-clinical study continue to be followed long-term.
- No drug related adverse events have been observed with either product.
About Feline Chronic Pain and Degenerative Joint Disease (DJD)
Degenerative joint disease, also known as osteoarthritis or degenerative arthritis, is one of the most significant and under-diagnosed diseases of cats. It involves the destruction of cartilage and supportive tissue which causes pain. 90% of cats over age 12 have radiographic evidence of joint disease. It is difficult for owners to recognize clinical symptoms of arthritis in a cat, as they often conceal their pain. Nearly 40% of all cats have clinical signs of DJD which are often mistaken for normal signs of aging. They include limping, difficulty with stairs, stiffness upon rising, pain when petted, and inability to jump.
Veterinarians spend considerable time educating their clients about chronic diseases such as DJD. Clients and patients – particularly cats – struggle with the need for regular treatments to manage chronic disease or pain. A pet’s limited ability to clearly communicate when they are experiencing early symptoms of their chronic conditions can prevent early intervention. These factors often result in both poor compliance and patient outcomes for chronic diseases. Scout Bio’s proprietary market data strongly supports the eagerness of veterinarians for one-time therapies that can effectively answer these challenges.
About Scout Bio
Scout Bio is a biotechnology company revolutionizing pet medicine by delivering a pipeline of one-time gene therapies for major chronic pet health conditions. Scout Bio’s therapeutics are designed to induce long-term expression of therapeutic proteins in pet patients using AAV vector technology. Scout Bio has a research and development collaboration with the University of Pennsylvania’s Gene Therapy Program, a leader in the field of genetic medicine research. Scout Bio is a private company headquartered in Philadelphia, Pennsylvania. For more information, see www.scoutbio.co.
For further information, please contact:
Fran Gaconnier
Scout Bio
214.417.4142
fran.gaconnier@scoutbio.co
Scout Bio Culture
At Scout Bio, we are building a team to deliver the future of veterinary medicine. We have a unique opportunity to transform quality-of-life for millions of pets around the world and their owners. To succeed in our mission, we need to grow and foster an exciting, rewarding work environment for our team. We want to grow with people who are nimble and resourceful, passionate about animals and excited by the prospect of working at the cutting edge of genetic medicine. We draw from a global talent pool and our culture reflects this diversity: there is no one way to think or solve problems!